
FDA cleared for autologous fat transfer
A new approach to restoring shape and appearance
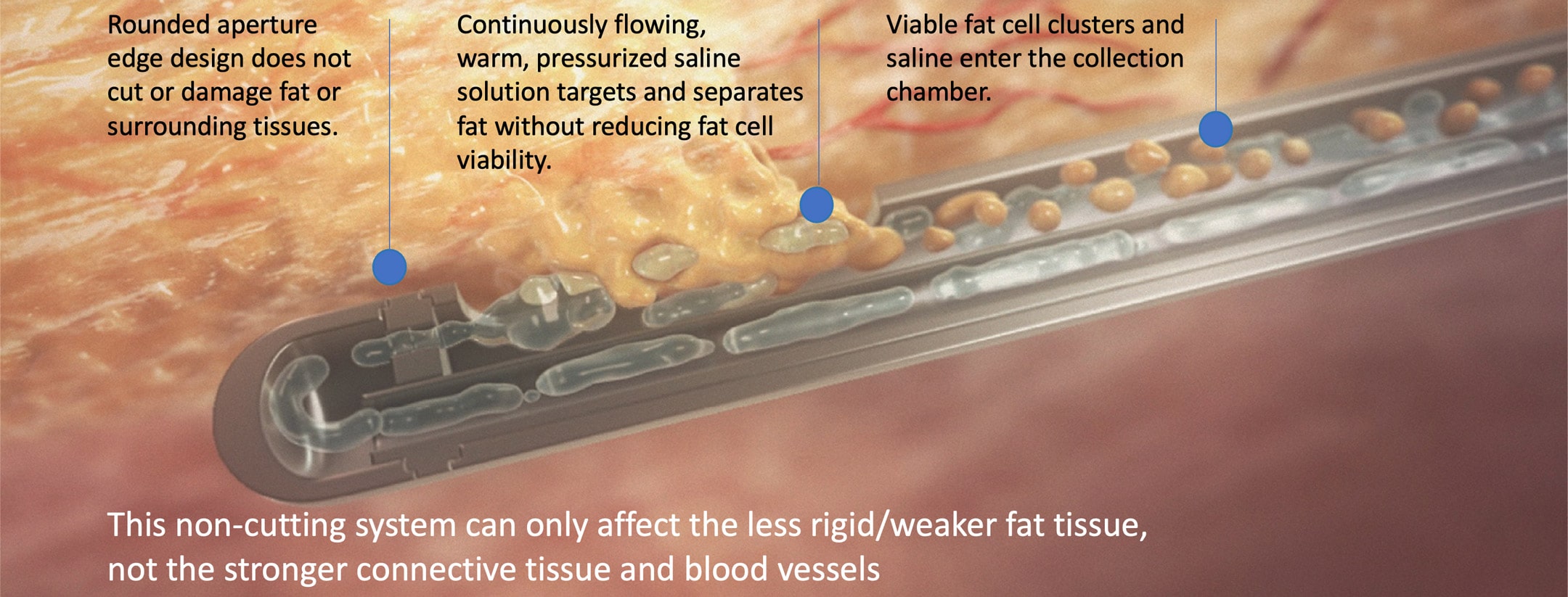
Selective Harvesting
Minimal Processing
Closed system for harvesting and processing
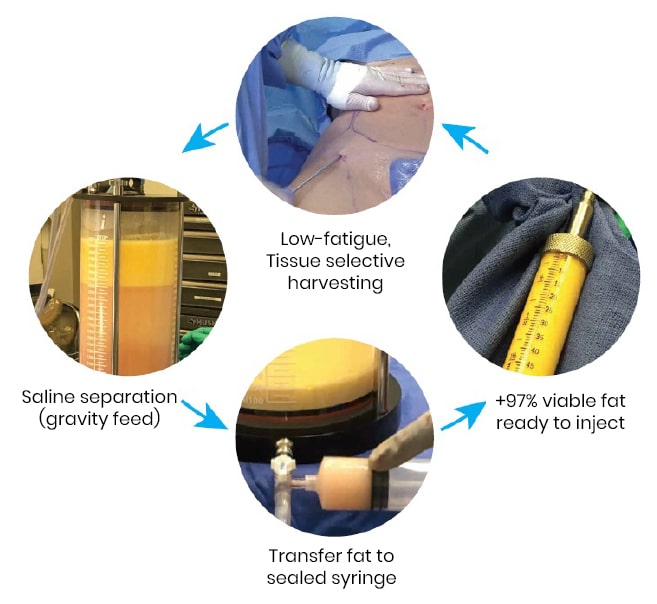
HydraSolve’s closed harvesting and processing provide efficient fat transfer procedures, eliminating the requirements of expensive and time-consuming processing systems. With its use of heat and pressure to separate and extract the fat, harvesting with HydraSolve is a very low-fatigue procedure. Once collected, simple gravity separation and decantation of the infranatant are all that are needed before transferring the fat to the syringe where it is ready for injection.
This minimized contamination risk and possible depreciation of fat cell viability from centrifuges or other separation methods.
HydraSolve produces high volumes of viable fat with minimal processing that can be used for breast reconstruction and lipofilling of the face, neck, hands, breast, and buttocks.
Highly Viable Fat
The results go well beyond ease of use:
- Fat that is over 97% viable Cell viability analysis of seven fresh tissue samples harvested from one live human subject and measured using Vision Cell Analyzer from Nexcelom Inc. Cell viability in four samples ranged from 94.4% to 99.2% an average of 96.6% compared to one sample harvested with UAL (Vaser) 72.7%, one sample harvested with SAL 82.7% and a sample harvested using Coleman syringe 85.5%.
- Cell Clusters are 0.5mm to 2.0mm in diameter, optimal for vascularization of the graft and re-injection ease.
